Document Type : Original Article
Authors
Department of Biology, Faculty of Basic Science, Damghan Branch, Islamic Azad University, Damghan, Iran
Abstract
Background: Laccases are a class of multi-copper oxidases that can catalyze the oxidation of various phenolic substrates while reduacing molecular oxygen to water. Although only a few bacterial laccases have been studied to date, recent advances in genome research suggest that these enzymes are widespread in bacteria. Due to their ability to oxidize a broad range of phenolic compounds, laccases have numerous biotechnological applications. The aim of this study was to isolate the gene encoding laccase (CotA) from recombinant Escherichia coli BL21 (DE3) containing the Bacillus licheniformis LS04 CotA-laccase gene and investigate its properties.
Methods: The bacterial strains, vectors, and growth conditions were used in the study, and also the recombinant and expression host strain construction was described. Plasmid isolation, PCR amplification, gel electrophoresis, and protein purification were also carried out. SDS-PAGE was used to visualize the protein bands and plasmid stability was analyzed. In addition, this study characterized the CotA laccase by evaluating its optimum temperature, pH, thermal stability, and activity after bathing at 50 °C for 10 min.
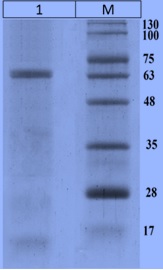
Results: The results showed that the CotA laccase produced a protein with a molecular weight of 65 kDa, and the plasmid was stable in the absence of antibiotic pressure for 200 generations. The pH profile for laccase activity showed a peak at pH 7.4, and the optimal temperature was found to be 45 °C. However, the pH and temperature stability of the CotA laccase was lower than that of the spore laccase.
Conclusion: The purified recombinant CotA-laccase showed high stability towards alkaline pH, high temperatures, and a broad pH range for catalyzing substrates. Nevertheless, the study demonstrates that CotA-laccase has the potential for industrial use due to its high stability and broad substrate range.
Graphical Abstract
Keywords
Main Subjects
OPEN ACCESS
©2023 The author(s). This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit: http://creativecommons.org/licenses/by/4.0/
PUBLISHER NOTE
Sami Publishing Company remains neutral concerning jurisdictional claims in published maps and institutional affiliations.
CURRENT PUBLISHER
- Giardina P, Faraco V, Pezzella C, Piscitelli A, Vanhulle S, Sannia G. Laccases: a never-ending story, Cell Mol Life Sci; 2010 Feb; 67:369-85. [Crossref], [Google Scholar], [Publisher]
- Singh G, Bhalla A, Kaur P, Capalash N, Sharma P. Laccase from prokaryotes: a new source for an old enzyme, Rev Environ Sci Biotechnol; 2011 Dec; 10:309-26. [Crossref], [Google Scholar], [Publisher]
- Koschorreck K, Richter SM, Ene AB, Roduner E, Schmid RD, Urlacher VB. Cloning and characterization of a new laccase from Bacillus licheniformis catalyzing dimerization of phenolic acids, Appl Microbiol Biotechnol; 2008 May; 79:217-24. [Crossref], [Google Scholar], [Publisher]
- Couto SR, Herrera JL. Industrial and biotechnological applications of laccases: a review, Biotechnol Adv; 2006 Sep 1; 24(5):500-13. [Crossref], [Google Scholar], [Publisher]
- Claus H. Laccases and their occurrence in prokaryotes, Arch Microbiol; 2003 Mar; 179:145-50. [Crossref], [Google Scholar], [Publisher]
- Sharma P, Goel R, Capalash N. Bacterial laccases, World J Microbiol Biotechnol; 2007 Jun; 23:823-32. [Crossref], [Google Scholar], [Publisher]
- Brissos V, Pereira L, Munteanu FD, Cavaco‐Paulo A, Martins LO. Expression system of CotA‐laccase for directed evolution and high‐throughput screenings for the oxidation of high‐redox potential dyes, Biotechnology Journal: Healthcare Nutrition Technology; 2009 Apr; 4(4):558-63. [Crossref], [Google Scholar], [Publisher]
- Roberts SA, Weichsel A, Grass G, Thakali K, Hazzard JT, Tollin G, Rensing C, Montfort WR. Crystal structure and electron transfer kinetics of CueO, a multicopper oxidase required for copper homeostasis in Escherichia coli, Proc Natl Acad Sci; 2002 Mar 5; 99(5):2766-71. [Crossref], [Google Scholar], [Publisher]
- Ruijssenaars HJ, Hartmans S. A cloned Bacillus halodurans multicopper oxidase exhibiting alkaline laccase activity, Appl Microbiol Biotechnol; 2004 Aug; 65:177-82. [Crossref], [Google Scholar], [Publisher]
- Miyazaki K. A hyperthermophilic laccase from Thermus thermophilus HB27, Extremophiles; 2005 Dec; 9:415-25. [Crossref], [Google Scholar], [Publisher]
- Koschorreck K, Schmid RD, Urlacher VB. Improving the functional expression of a Bacillus licheniformislaccase by random and site-directed mutagenesis, BMC Biotechnol; 2009 Dec; 9(1):1-0. [Crossref], [Google Scholar], [Publisher]
- Dalfard AB, Khajeh K, Soudi MR, Naderi-Manesh H, Ranjbar B, Sajedi RH. Isolation and biochemical characterization of laccase and tyrosinase activities in a novel melanogenic soil bacterium, Enzyme Microb Technol; 2006 Nov 3; 39(7):1409-16. [Crossref], [Google Scholar], [Publisher]
- Muthukumarasamy NP, Jackson B, Joseph Raj A, Sevanan M. Production of extracellular laccase from Bacillus subtilis MTCC 2414 using agroresidues as a potential substrate, Biochem Res Int; 2015 Sep 14; 2015. [Crossref], [Google Scholar], [Publisher]
- Xu KZ, Wang HR, Wang YJ, Xia J, Ma H, Cai YJ, Liao XR, Guan ZB. Enhancement in catalytic activity of CotA-laccase from Bacillus pumilus W3 via site-directed mutagenesis, J Biosci Bioeng; 2020 Apr 1; 129(4):405-11. [Crossref], [Google Scholar], [Publisher]
- Tarasov A, Stozhko N, Bukharinova M, Khamzina E. Biosensors based on phenol oxidases (laccase, tyrosinase, and their mixture) for estimating the total phenolic index in food-related samples, Life; 2023 Jan 20; 13(2):291. [Crossref], [Google Scholar], [Publisher]
- Bhatt P, Bhatt K, Chen WJ, Huang Y, Xiao Y, Wu S, Lei Q, Zhong J, Zhu X, Chen S. Bioremediation potential of laccase for catalysis of glyphosate, isoproturon, lignin, and parathion: Molecular docking, dynamics, and simulation, J Hazard Mater; 2023 Feb 5; 443:130319. [Crossref], [Google Scholar], [Publisher]
- Fillat Ú, Ibarra D, Eugenio ME, Moreno AD, Tomás-Pejó E, Martín-Sampedro R. Laccases as a potential tool for the efficient conversion of lignocellulosic biomass: a review, Fermentation; 2017 May 2; 3(2):17. [Crossref], [Google Scholar], [Publisher]
- Bassanini I, Ferrandi EE, Riva S, Monti D. Biocatalysis with laccases: An updated overview, Catalysts; 2020 Dec 28; 11(1):26. [Crossref], [Google Scholar], [Publisher]
- Liu Y, Chen Z, Ng TB, Zhang J, Zhou M, Song F, et al. (2007). Bacisubin, an antifungal protein with ribonuclease and hemagglutinating activities from Bacillus subtilis strain B-916. Peptides, 28(3):553-559. [Crossref], [Google Scholar], [Publisher]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding, Anal Biochem; 1976 May 7; 72(1-2):248-54. [Crossref], [Google Scholar], [Publisher]
- Liu Y, Lu F, Chen G, Snyder CL, Sun J, Li Y, Wang J, Xiao J. High-level expression, purification and characterization of a recombinant medium-temperature α-amylase from Bacillus subtilis, Biotechnol Lett; 2010 Jan; 32:119-24. [Crossref], [Google Scholar], [Publisher]
- Chen B, Xu WQ, Pan XR, Lu L. A novel non-blue laccase from Bacillus amyloliquefaciens: secretory expression and characterization, Int J Biol Macromol; 2015 May 1; 76:39-44. [Crossref], [Google Scholar], [Publisher]
- Fang Z, Li T, Wang Q, Zhang X, Peng H, Fang W, Hong Y, Ge H, Xiao Y. A bacterial laccase from marine microbial metagenome exhibiting chloride tolerance and dye decolorization ability, Appl Microbiol Biotechnol; 2011 Feb; 89:1103-10. [Crossref], [Google Scholar], [Publisher]
- Jim
|
enez-Juarez N, Roman-Miranda R, Baeza A, Sánchez-Amat A, Vazquez-Duhalt R, Valderrama B. Alkali and halide-resistant catalysis by the multipotent oxidase from Marinomonas mediterranea, J biotechnol; 2005 Apr 20;117(1):73-82. [Crossref], [Google Scholar], [Publisher]
- McMahon AM, Doyle EM, Brooks S, O’Connor KE. Biochemical characterisation of the coexisting tyrosinase and laccase in the soil bacterium Pseudomonas putida F6, Enzyme Microb Technol; 2007 Apr 3; 40(5):1435-41. [Crossref], [Google Scholar], [Publisher]
- Wu J, Kim KS, Lee JH, Lee YC. Cloning, expression in Escherichia coli, and enzymatic properties of laccase from Aeromonas hydrophila WL-11, J Environ Sci; 2010 Jan 1; 22(4):635-40. [Crossref], [Google Scholar], [Publisher]
- Cho EA, Seo J, Lee DW, Pan JG. Decolorization of indigo carmine by laccase displayed on Bacillus subtilis spores, Enzyme Microb Technol; 2011 Jun 10; 49(1):100-4. [Crossref], [Google Scholar], [Publisher]